Chemical reactions: Chemical equation, Balanced chemical equation, implications of a balanced chemical equation, types of chemical reactions: combination, decomposition, displacement, double displacement, precipitation, neutralization, oxidation and reduction.
Types of Reactions
What is a chemical change?
Writing chemical equations
| |||||||||||||||||||||||||||||||
|
There
are four main types of reactions:
Combination Reactions
Decomposition Reactions
Single-Replacement Reactions
Double-Replacement Reactions
Combination Reaction
A combination reaction combines two or more reactants in to a single product.
The general
pattern for this reaction is:
Examples of
substances that often combine in synthesis reactions:
Decomposition
Reaction
Decomposition
reactions break down, or “decompose” a single reactant into multiple products.
The general
pattern for decomposition reactions:
“AB” is
always a compound
A and B
(products) may be single elements or smaller compounds
Some common
types of decomposition reactions:
Single-replacement Reaction
In a
single-replacement reaction, a reactive element replaces a less reactive
element in a compound.
The general
pattern for single-replacement reactions:
A + BX à B + AX
A =
reactive element
The B in BX
is less reactive than A.
Common
single-replacement reactions:
*More
active metal replaces the less active:
Zn (s) +
CuCl2 (aq) à Cu
(s) + ZnCl2 (aq)
*Metal
replaces the H in an acid:
Mg (s) +
2HCl (aq) à MgCl2 + H2 (g)
*More
active halogen replaces less reactive:
Cl2 (g) +
2NaBr (aq) à
2NaCl + Br2
Single-replacement
reactions occur because the element that does the replacing is a more active
element, based on its tendency to lose or gain electrons.
If a
compound is placed in contact with a more active element, a reaction is likely.
To
determine whether or not a single-replacement reaction will occur, refer to the
“activity series.”
If the free
element is above the other (in the compound) on the activity series, a reaction
will occur.
If the free
element is below the other on the activity series, a reaction will not occur.
Will a
Reaction Occur?
Au (s) +
NaCl (aq) à ?
Zn (s) +
H2SO4 à ?
Cl2 (g) +
MgBr2 à ?
Mg (s) +
Ca(OH)2 à ?
Double-Replacement Reactions
In a
double-replacement reaction, two compounds switch partners.
The general
pattern of this reaction:
AY + BX à AX + BY
Most
double-replacement reactions take place when both reactants are aqueous
(dissolved in water).
When two
aqueous compounds are mixed, and partners “swap,” a precipitate is often
formed.
Example:
Pb(NO3)2
(aq) + K2CrO4 (aq) à
PbCrO4 (s) + 2KNO3 (aq)
Another
example double-replacement reaction:
HCl (aq) +
KOH (aq) à HOH (l) + KCl (aq)
If we break
apart the compounds into ions, we get:
H+ + Cl- +
K+ + OH- à HOH + K+ + Cl-
**This is
called the “ionic equation.”
H+ (aq) +
Cl- (aq) + K+ (aq) + OH- (aq) à HOH (l) + K+ (aq) + Cl- (aq)
K+ and Cl-
are “spectator ions”
If you
cross out what does not change from reactants to products (spectator ions), you
get the “net ionic equation.”
H+ + OH- à HOH
Balanced and unbalanced chemical equations:
A balanced chemical equation has an equal number of atoms of different elements in the reactants and products.
Zinc metal reacts with dilute sulphuric acid to form zinc sulphate and hydrogen gas.
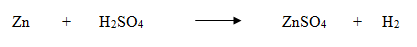
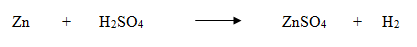
Count the number of atoms of all the elements in the reactants and products separately.
In reactants
|
In products
| |
No. of Zn atoms
|
1
|
1
|
No. of H atoms
|
2
|
2
|
No. of S atoms
|
1
|
1
|
No. of O atoms
|
4
|
4
|
Characteristics of Chemical reactions
- The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be easily observed easily.
- The important characteristics of chemical reactions are:
- Evolution of a gas
- Formation of a precipitate
- Change in colour
- Change in temperature
- Change in state
Evolution of a Gas:
Some chemical reactions are characterized by the evolution of a gas.
The chemical reaction between zinc and dilute sulphuric acid is characterized by the evolution of hydrogen gas.
- Take some zinc granules in a conical flask.
- Add dilute sulphuric acid over zinc granules.
- We will see the bubbles of hydrogen gas being formed around zinc granules.
- If we touch the conical flask with our hand, we will find that it is somewhat hot. So, a change in temperature also occurs in this chemical reaction.
Formation of a precipitate:
Some chemical reactions are characterized by the formation of precipitate. A precipitate is a ‘solid product’ which separates out from the solution during a chemical reaction.
The chemical between potassium iodide and lead nitrate is characterized by the formation of a yellow precipitate of lead iodide.
- Take some lead nitrate solution in test tube.
- Add potassium iodide solution to it.
- A yellow precipitate of lead iodide is formed at once.
- A change in colour also takes place in this chemical reaction.
Change in colour:
Some chemical reactions are characterized by a change in colour.
The chemical reaction between citric acid and purple coloured potassium permanganate solution is characterized by a change in colour from purple to colourless.
- Take some dilute potassium permanganate solution in a test tube. It has purple colour.
- Add some lemon juice (it contains citric acid) to it with the help of a dropper and shake the test tube.
- The purple colour of potassium permanganate solution goes on fading and ultimately it becomes colourless.
Change in temperature:
Some chemical reactions are characterized by a change in temperature.
The chemical reaction between quicklime and water to form slaked lime is characterized by a change in temperature.
- Take a little of quicklime in a hard-glass beaker.
- Add water to it slowly.
- Touch the beaker.
- The beaker feels to be quite hot.
Change in state:
- Some chemical reactions are characterized by a change in state.
- When wax is burned (in the form of wax candle), then water and carbon dioxide are formed.
- Now, wax is a liquid whereas carbon dioxide is a gas. This means that during the combustion reaction of wax, the physical state changes from solid to liquid and gas.
Chemical equations:
- The method of representing a chemical reaction with the help of symbols and formulae of the substances involved in it is known as a chemical equation.
- Zinc metal reacts with dilute sulphuric acid to form zinc sulphate and hydrogen gas.
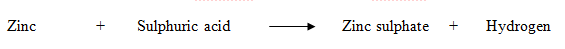
- This is known as word equation. Putting the symbols and formulae of all the substances in the above word equation, we get the following chemical equation:
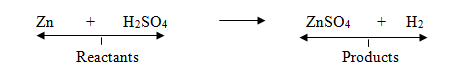
- The substances which combine or react are known as reactants.
- The new substances produced in a reaction are known as products.
- A chemical equation is a short-hand method of representing a chemical reaction.
Oxidation and Reduction reactions:
- Oxidation:
- The addition of oxygen to a substance is called oxidation.
- The removal of hydrogen from a substance is called oxidation.
- Reduction:
- The addition of hydrogen to a substance is called reduction.
- The removal of oxygen from a substance is called reduction.
- The oxidation and reduction reactions are also called redox reactions.Example:When copper oxide is heated with hydrogen, then copper metal and water are formed.
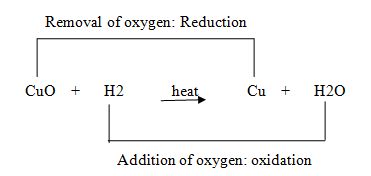 In the above reaction, copper oxide (CuO) is changing into copper (Cu), so copper oxide is being reduced to copper.Hydrogen is changing into water (H2O), so hydrogen is being oxidized to water.Copper oxide is giving oxygen required for the oxidation of hydrogen, therefore, copper oxide is oxidizing agent and hydrogen is reducing agent.
In the above reaction, copper oxide (CuO) is changing into copper (Cu), so copper oxide is being reduced to copper.Hydrogen is changing into water (H2O), so hydrogen is being oxidized to water.Copper oxide is giving oxygen required for the oxidation of hydrogen, therefore, copper oxide is oxidizing agent and hydrogen is reducing agent. - Effect of oxidation reactions in everyday life:Oxidation has damaging effect on metals as well as on food. There are two common effects of oxidation reactions which we observe in daily. These are:
- Corrosion of metals:Corrosion is the process in which metals are eaten up gradually by the action of air, moisture or a chemical (such as an acid) on their surface.
- Corrosion is caused mainly by the oxidation of metals by oxygen of air. Rusting of iron metal is the most common form of corrosion.
- During the corrosion of iron (rusting of iron), iron metal is oxidized by the oxygen of air in the presence of water (moisture) to form hydrated iron (III) oxide called rust.

- Corrosion weakens the iron and steel objects and structures such as railings, car bodies, bridges and ships, etc., and cuts short their life.
- Rancidity
- When the fats and oils present in food materials get oxidized by the oxygen (of air), their oxidation products have unpleasant smell and taste.
- The condition produced by aerial oxidation of fats and oils in foods marked by unpleasant smell and taste is called rancidity.
- Rancidity spoils the food materials prepared in fats and oils which have been kept for a considerable time and make them unfit for eating.
- The development of rancidity of food can be prevented or retarded (slowed down) in the following ways:
- Rancidity can be prevented by adding anti-oxidants to foods containing fats and oils:Anti-oxidant is a substance (or chemical) which prevents oxidation. Anti-oxidants are actually reducing agent.The two common anti-oxidants used in foods to prevent the development of rancidity are BHA (Butylated Hydroxy – Anisole) and BHT (Butylated Hydroxy-Toluene).
- Rancidity can be prevented by packaging fats and oils containing foods in nitrogen gas:When the packed is surrounded by unreactive gas nitrogen, there is no oxygen to cause its oxidation and make it rancid.The manufacturers of potato chips fill the plastic bags containing chips with nitrogen gas.
- Rancidity can be retarded by keeping food in a refrigerator.
- Rancidity can be retarded by storing food in air-tight containers.
- Rancidity can be retarded by storing food away from light.


No comments:
Post a Comment