Molecule
A molecule (/ˈmɒlɪkjuːl/) is an electrically neutral group of two or more atoms held together by chemical bonds.[3][4][5][6][7] Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the termmolecule is often used less strictly, also being applied to polyatomic ions.
In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. According to this definition, noble gas atoms are considered molecules as they are in fact monoatomic molecules.
A molecule may be homonuclear, that is, it consists of atoms of a single chemical element, as with oxygen (O2); or it may beheteronuclear, a chemical compound composed of more than one element, as with water (H2O). Atoms and complexes connected bynon-covalent bonds such as hydrogen bonds or ionic bonds are generally not considered single molecules.
Molecules as components of matter are common in organic substances (and therefore biochemistry). They also make up most of the oceans and atmosphere. However, the majority of familiar solid substances on Earth, including most of the minerals that make up thecrust, mantle, and core of the Earth, contain many chemical bonds, but are not made of identifiable molecules. Also, no typical molecule can be defined for ionic crystals (salts) and covalent crystals (network solids), although these are often composed of repeating unit cellsthat extend either in a plane (such as in graphene) or three-dimensionally (such as in diamond, quartz, or sodium chloride). The theme of repeated unit-cellular-structure also holds for most condensed phases with metallic bonding, which means that solid metals are also not made of molecules. In glasses (solids that exist in a vitreous disordered state), atoms may also be held together by chemical bonds without presence of any definable molecule, but also without any of the regularity of repeating units that characterizes crystals.
Molecular science
The science of molecules is called molecular chemistry or molecular physics, depending on whether the focus is on chemistry or physics. Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties. In practice, however, this distinction is vague. In molecular sciences, a molecule consists of a stable system (bound state) composed of two or more atoms.Polyatomic ions may sometimes be usefully thought of as electrically charged molecules. The term unstable molecule is used for very reactive species, i.e., short-lived assemblies (resonances) of electrons and nuclei, such as radicals, molecular ions, Rydberg molecules, transition states, van der Waals complexes, or systems of colliding atoms as in Bose–Einstein condensate.
History and etymology
According to Merriam-Webster and the Online Etymology Dictionary, the word "molecule" derives from the Latin "moles" or small unit of mass.
- Molecule (1794) – "extremely minute particle", from French molécule (1678), from New Latin molecula, diminutive of Latin moles "mass, barrier". A vague meaning at first; the vogue for the word (used until the late 18th century only in Latin form) can be traced to the philosophy of Descartes.[10][11]
The definition of the molecule has evolved as knowledge of the structure of molecules has increased. Earlier definitions were less precise, defining molecules as the smallestparticles of pure chemical substances that still retain their composition and chemical properties.[12] This definition often breaks down since many substances in ordinary experience, such as rocks, salts, and metals, are composed of large crystalline networks of chemically bonded atoms or ions, but are not made of discrete molecules.
Bonding
Molecules are held together by either Covalent Bonding or Ionic Bonding. Several types of non metal elements exist only as molecules in the environment. For example, Hydrogen only exists as Hydrogen molecule. A molecule of a compound is made out of two or more elements.
Covalent
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
Ionic
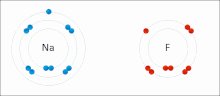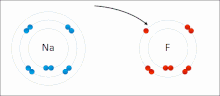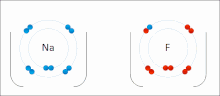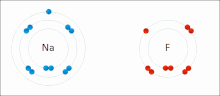
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. The ions are atoms that have lost one or more electrons (known as cations) and atoms that have gained one or more electrons (known as anions). This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like NH4+ or SO42−. In simpler words, an ionic bond is the transfer of electrons from a metal to a non-metal in order for both atoms to obtain a full valence shell.
Molecular size
Most molecules are far too small to be seen with the naked eye, but there are exceptions. DNA, a macromolecule, can reach macroscopicsizes, as can molecules of many polymers. Molecules commonly used as building blocks for organic synthesis have a dimension of a fewangstroms (Å) to several dozen Å, or around one billionth of a meter. Single molecules cannot usually be observed by light (as noted above), but small molecules and even the outlines of individual atoms may be traced in some circumstances by use of an atomic force microscope. Some of the largest molecules are macromolecules or supermolecules.
Effective molecular radius is the size a molecule displays in solution.[17][18] The table of permselectivity for different substances contains examples.
Molecular formulas
Chemical formula types
The chemical formula for a molecule uses a single line of chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts.
A compound's empirical formula is a very simple type of chemical formula. It is the simplest integer ratio of the chemical elements that constitute it. For example, water is always composed of a 2:1 ratio of hydrogen to oxygen atoms, and ethyl alcohol or ethanol is always composed of carbon, hydrogen, and oxygen in a 2:6:1 ratio. However, this does not determine the kind of molecule uniquely – dimethyl ether has the same ratios as ethanol, for instance. Molecules with the same atoms in different arrangements are called isomers. Also carbohydrates, for example, have the same ratio (carbon:hydrogen:oxygen = 1:2:1) (and thus the same empirical formula) but different total numbers of atoms in the molecule.
The molecular formula reflects the exact number of atoms that compose the molecule and so characterizes different molecules. However different isomers can have the same atomic composition while being different molecules.
The empirical formula is often the same as the molecular formula but not always. For example, the molecule acetylene has molecular formula C2H2, but the simplest integer ratio of elements is CH.
The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 1/12 of the mass of a neutral carbon-12 (12Cisotope) atom. For network solids, the term formula unit is used in stoichiometric calculations.
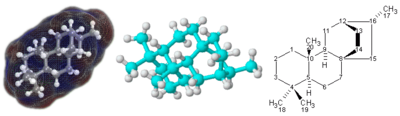
Structural formula
For molecules with a complicated 3-dimensional structure, especially involving atoms bonded to four different substituents, a simple molecular formula or even semi-structural chemical formula may not be enough to completely specify the molecule. In this case, a graphical type of formula called a structural formula may be needed. Structural formulas may in turn be represented with a one-dimensional chemical name, but suchchemical nomenclature requires many words and terms which are not part of chemical formulas.
Molecular geometry
Molecules have fixed equilibrium geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity. Isomers share a chemical formula but normally have very different properties because of their different structures. Stereoisomers, a particular type of isomer, may have very similar physico-chemical properties and at the same time different biochemical activities.
Molecular spectroscopy
Molecular spectroscopy deals with the response (spectrum) of molecules interacting with probing signals of known energy (orfrequency, according to Planck's formula). Molecules have quantized energy levels that can be analyzed by detecting the molecule's energy exchange through absorbance or emission.[21] Spectroscopy does not generally refer to diffraction studies where particles such asneutrons, electrons, or high energy X-rays interact with a regular arrangement of molecules (as in a crystal).
Microwave spectroscopy commonly measures changes in the rotation of molecules, and can be used to identify molecules in outer space.Infrared spectroscopy measures changes in vibration of molecules, including stretching, bending or twisting motions. It is commonly used to identify the kinds of bonds or functional groups in molecules. Changes in the arrangements of electrons yield absorption or emission lines in ultraviolet, visible or near infrared light, and result in colour. Nuclear resonance spectroscopy actually measures the environment of particular nuclei in the molecule, and can be used to characterise the numbers of atoms in different positions in a molecule.
Theoretical aspects
The study of molecules by molecular physics and theoretical chemistry is largely based on quantum mechanics and is essential for the understanding of the chemical bond. The simplest of molecules is the hydrogen molecule-ion, H2+, and the simplest of all the chemical bonds is the one-electron bond. H2+ is composed of two positively charged protons and one negatively charged electron, which means that the Schrödinger equation for the system can be solved more easily due to the lack of electron–electron repulsion. With the development of fast digital computers, approximate solutions for more complicated molecules became possible and are one of the main aspects of computational chemistry.
When trying to define rigorously whether an arrangement of atoms is "sufficiently stable" to be considered a molecule, IUPAC suggests that it "must correspond to a depression on the potential energy surface that is deep enough to confine at least one vibrational state".[3]This definition does not depend on the nature of the interaction between the atoms, but only on the strength of the interaction. In fact, it includes weakly bound species that would not traditionally be considered molecules, such as the helium dimer, He2, which has one vibrational bound state[22] and is so loosely bound that it is only likely to be observed at very low temperatures.
Whether or not an arrangement of atoms is "sufficiently stable" to be considered a molecule is inherently an operational definition. Philosophically, therefore, a molecule is not a fundamental entity (in contrast, for instance, to an elementary particle); rather, the concept of a molecule is the chemist's way of making a useful statement about the strengths of atomic-scale interactions in the world that we observe.





No comments:
Post a Comment